Marvin EDC

Marvin EDC
About Marvin EDC
Marvin EDC is a clinical data management software designed to help businesses in pharmaceutical, biotech, medical, and other industries manage customers, clinical trials, inventories, and more. The electronic data capture (EDC) module allows organizations to collect and verify data from various online sources and handle compliance tracking operations.
It offers a host of features including access control, notifications, reporting, data cleansing, electronic signature capture, data backup, workflow management, and more. Health professionals can use the application to manage double data entry (DDE) operations, view audit trail, and store eClinical documents in a centralized database. Marvin EDC also offers multiple randomization methods for executing clinical trials such as open or double blind, multi-group, multi-strata, and more based on variance minimization or block lists.
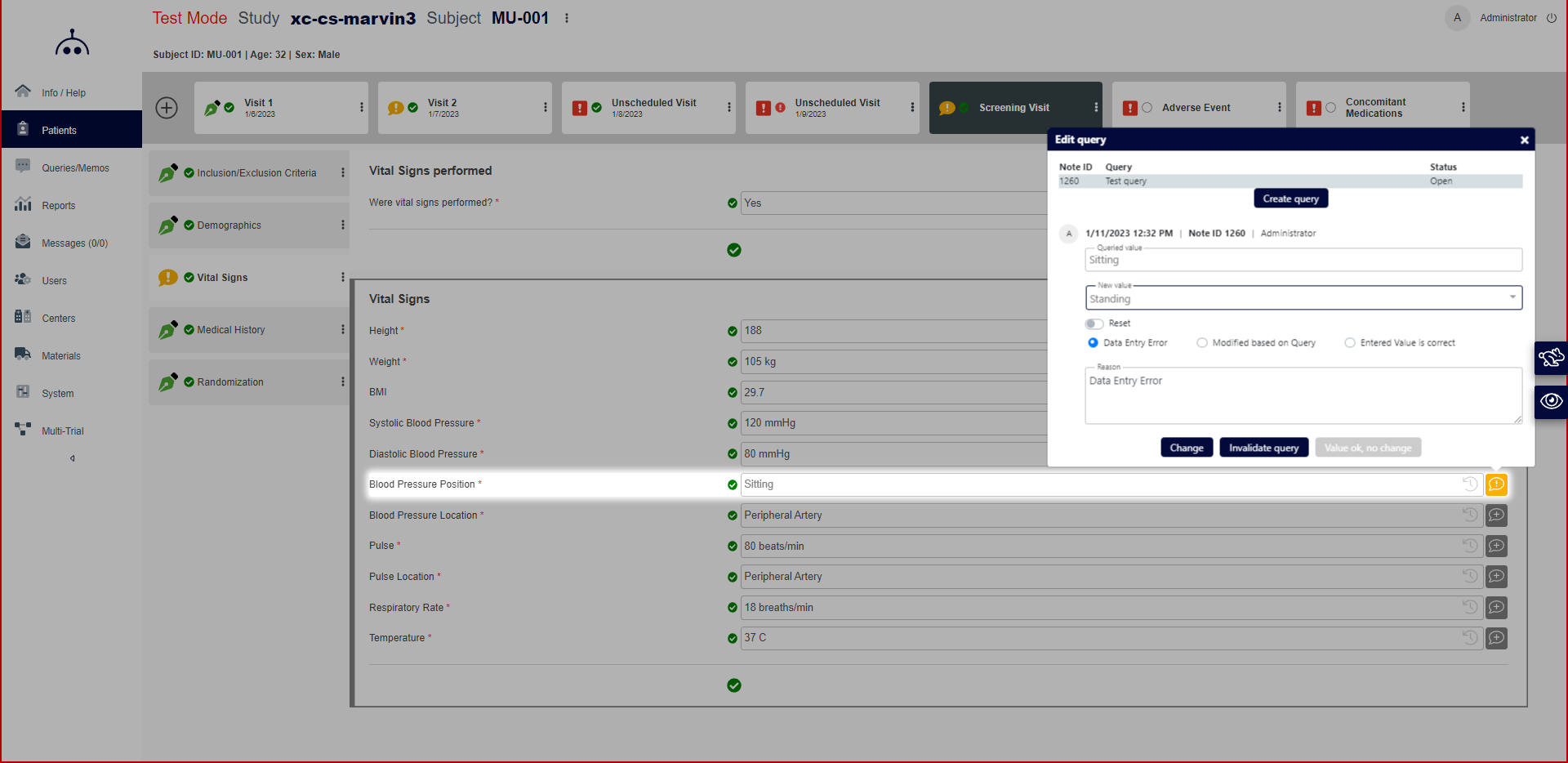
Marvin EDC includes a materials accountability module, which enables administrators to monitor stock levels and track shipments of materials from a centralized warehouse to different sites using package verification numbers. It lets healthcare workers streamline source data verification (SDV) processes by assessing risk levels for specific study centers and defining validation rules.
Key benefits of Marvin EDC
1. Efficient Study Setup and Management:
Easy Study Setup: Marvin EDC is designed for straightforward study setup, making it easier to get clinical trials up and running.
Flexible and Adaptive: The system is adaptable to various study types and supports different languages and browsers.
Automated Documentation: Marvin can automatically generate documentation like annotated CRFs and DVP.
Efficient Amendment Management: It streamlines the process of handling mid-study amendments with automatic version control.
2. Robust Data Management Features:
Centralized Data Collection: Marvin provides a centralized platform for data capture, storage, retrieval, and organization.
Real-time Data Access: It allows authorized users to access data in real-time, improving collaboration and efficiency.
Data Cleansing and Validation: Marvin includes features for data cleansing and validation, ensuring data quality.
Advanced Query Management: It facilitates efficient query management to address data inconsistencies.
Electronic Signature Capture: Supports electronic signatures for data verification.
Audit Trails: Provides detailed audit trails for tracking data changes and user activity.
Materials Accountability: A materials accountability module helps manage and track materials, including shipments.
3. Enhanced Data Quality and Integrity:
Risk-Based Monitoring: Marvin supports risk-based monitoring, allowing for customized verification rules based on risk levels.
Double Data Entry (DDE): It supports DDE for paper and hybrid studies, with automatic verification and discrepancy highlighting.
Data Backup and Archiving: Supports data backup and electronic archiving in multiple formats.
Integration Capabilities: Marvin offers web service APIs for integration with other systems.
4. Cost and Time Savings:
Reduced Administrative Tasks: Automating data management processes reduces the need for manual data entry and associated costs.
Faster Database Setup: Tools eliminate the need for programming, speeding up database setup.
Improved Efficiency: The system's features contribute to faster clinical trial timelines and reduced costs.
Images
Not sure about Marvin EDC?
Compare with a popular alternative
Show more details
Starting Price
Pricing Options
Features
Integrations
Ease of Use
Value for Money
Customer Service
Alternatives
Filter by
Time used
Industry
3 Reviews
This service may contain translations provided by google. Google disclaims all warranties related to the translations, express or implied, including any warranties of accuracy, reliability, and any implied warranties of merchantability, fitness for a particular purpose and noninfringement. Gartner's use of this provider is for operational purposes and does not constitute an endorsement of its products or services.
- Industry: Research
- Company size: 11–50 Employees
- Used Daily for 2+ years
-
Review Source
Show more details
Overall rating
- Ease of Use
- Customer Support Software
- Likelihood to recommend 10.0 /10
Recommendable EDC system
Reviewed on 2020-10-28
Pros
The fact the system is flexible and customizable and offers many possibilities.
Cons
System documentation is too technical especially for beginners.
Response from EvidentIQ
Dear Beate, thank you for your positive review. It's great to hear that you enjoy the flexibility of Marvin. We are happy to have you as a long term customer. Good luck further with your many studies. We hope to see you again in 2021, Sabine Birkner, Marketing Manager XC Munich
- Industry: Pharmaceuticals
- Company size: 51–200 Employees
- Used Daily for 2+ years
-
Review Source
Show more details
Overall rating
- Value for Money
- Ease of Use
- Customer Support Software
- Likelihood to recommend 9.0 /10
Powerful EDC software
Reviewed on 2020-11-02
Pros
- Lean and intuitive user interface
- Fully CDISC-ODM compliant
- Easy to use development tools
- Excellent customer support
Cons
- Configuration of the Permission Framework is complex
- Few Integrated standard reports with limited scope
- Industry: Pharmaceuticals
- Company size: 51–200 Employees
- Used Daily for 2+ years
-
Review Source
Show more details
Overall rating
- Ease of Use
- Customer Support Software
- Likelihood to recommend 7.0 /10
MARVIN: 10 years of use
Reviewed on 2020-10-29
Pros
The feedback received from end user regarding MARVIN is that it's an user friendly system. We think that MARVIN is a very flexible (it allows us to manage very complex amendment and study design). It's also very easy to integrate with external parties (using real web services and the REST technology).
Cons
An improvement of the reporter tool is needed.
Marvin EDC FAQs
Below are some frequently asked questions for Marvin EDC.Q. What type of pricing plans does Marvin EDC offer?
Marvin EDC offers the following pricing plans:
- Starting from: US$0.05
- Free Trial: Available
Please contact EvidentIQ Germany directly for pricing information.
Q. Who are the typical users of Marvin EDC?
Marvin EDC has the following typical customers:
Self Employed, 2–10, 11–50, 51–200, 201–500, 501–1,000
Q. What languages does Marvin EDC support?
Marvin EDC supports the following languages:
Chinese, Dutch, English, French, German, Italian, Japanese, Spanish
Q. Does Marvin EDC support mobile devices?
Marvin EDC supports the following devices:
Q. What other apps does Marvin EDC integrate with?
We do not have any information about what integrations Marvin EDC has
Q. What level of support does Marvin EDC offer?
Marvin EDC offers the following support options:
Email/Help Desk, Knowledge Base Software, Phone Support, 24/7 (Live rep), Chat
Related categories
See all software categories found for Marvin EDC.



